Eye Drop Recall:
Eye drop lubricants are extremely common over-the-counter eye products used to keep the eye moist and reduce the symptoms of dry eye disease.
In February 2023, upon the U.S. Food and Drug Administration’s recommendation to recall Delsam Pharma’s Artificial Eye Ointment, Global Pharma initiated a voluntary recall.
Sadly, the death toll and the number of people who have lost their vision linked to these recalled eye drops has risen. Some individuals have needed to have their eyeballs surgically removed. The Centers for Disease Control and Prevention (CDC) reported on March 21, 2023 that three people have died, eight have reported permanent vision loss, and four have needed enucleation (surgical removal of the eyeball).
Find out if you have a case
See If You Qualify for a
Eye Drop Contamination Lawsuit
*The information you send is 100% confidential.
Contaminated Eye Drops Lead to Vision Loss, Deaths: File a Claim Now
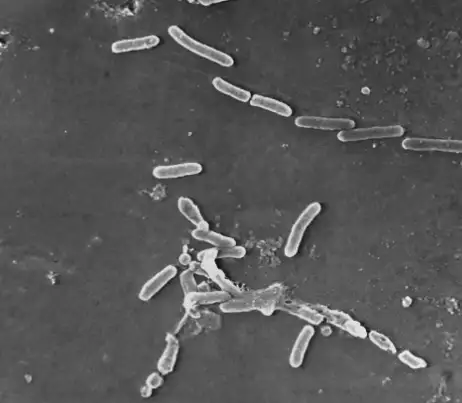
In early February, the FDA issued a warning to consumers and health care providers to cease the purchase and use of EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears due to possible bacterial contamination.
Despite these over-the-counter products being manufactured with the intention to be sterile, the CDC has identified 68 patients in 16 states who are infected with Pseudomonas aerugionosa. What makes this bacteria all the more dangerous is that it is resistant to most antibiotics.
In recent months, over ten brands have had their artificial tears products recalled. However, a majority of the cases have been linked to the eye drop brands EzriCare and Delsam Pharama, eye drops that were manufactured by Global Pharma Healthcare.
As previously mentioned, the FDA recommended a recall of these eye drops due to Global Pharma’s CGMP (current good manufacturing practice) violations. These violations included a lack of appropriate microbial testing, a lack of proper control concerning tamper-evident packaging, and formulation issues.
Dangers of Antibiotic-Resistant Bacteria
The type of bacteria found in the eye drops was Pseudomonas aeruginosa. It is most commonly found in the soil, water, and on the hands of healthy people. People with weakened immune systems are often most at risk of infection, particularly in common health care settings such as hospitals.
The CDC has reported that before this outbreak, this particular drug-resistant strain had never been seen in America.
It was noted that not all affected patients had eye infections. Others had developed respiratory or urinary tract infections. The drug-resistant bacteria is fatal when it enters the bloodstream.
Are You Affected? Symptoms of Eye Infection
The CDC urges people to seek medical care if they experience any of the following symptoms:
- Increased light sensitivity
- Blurry vision
- Eye pain or discomfort
- Yellow, green, or clear discharge from the eye
- Redness of the eye or eyelid
- The feeling of a foreign object in the eye (foreign body sensation)
EzriCare and Delsam Eye Drop Lawsuit
 Numerous individuals have filed lawsuits against EzriCare and Delsam Pharma due to the eye infections brought about by the contaminated eye drops.
Numerous individuals have filed lawsuits against EzriCare and Delsam Pharma due to the eye infections brought about by the contaminated eye drops.
These victims have filed class-action lawsuits as well as individual injury lawsuits. Plaintiffs of class action lawsuits are seeking refunds for the purchase of these eye-care products. Plaintiffs of individual injury lawsuits however are seeking compensation for their bacterial infections and eye problems.
If you or a loved one have experienced any of the previously mentioned symptoms after using these eye drops, sustained a bacterial eye infection, or developed eye problems due to the contaminated eye drops, you must take action against the at-fault party.
Find out if you have a case
Eye Drop Recall Status
Although the FDA has yet to issue a recall classification for EzriCare and Delsam Pharma artificial tears, their status with the agency remains active.
The CDC and the FDA are working together to investigate the multi-state outbreak. They are currently assessing whether carbapenem-resistant Pseudomonas aeruginosa (CRPA) is present in any sealed eye drop bottles of artificial tears.
In a statement, CDC and FDA recommended clinicians and patients to stop using EzriCare or Delsam Pharma’s Artificial Tears products pending additional guidance.
Clinicians have been instructed to report any CRPA case from an ocular specimen gathered since 2022 to the local or state health department. They can also contact the CDC for assistance in submitting specimens.
What are the Possible Recoverable Damages in the EzriCare Eye Drop Lawsuit?
 If you have been the victim of EzriCare Artificial Tears or Delsam Pharma’s Artificial Eye Ointment, you may be eligible to file a personal injury claim or lawsuit to recover compensation for the injuries and damages you sustained.
If you have been the victim of EzriCare Artificial Tears or Delsam Pharma’s Artificial Eye Ointment, you may be eligible to file a personal injury claim or lawsuit to recover compensation for the injuries and damages you sustained.
When filing a claim, there are several factors that are taken into consideration when calculating the value of a claim.
Here are some of the factors that can affect the amount of compensation you may be entitled to:
- The severity of your injuries and any resulting disabilities
- Lost wages, including both past and future earnings
- Medical expenses stemming from the event, such as hospital bills, doctor visits, physical therapy treatments, medication costs, and more
- Pain and suffering caused by the incident (physical pain, mental anguish, etc.)
- Loss of consortium or companionship due to a spouse, family member, or close friend being injured in the incident.
It is crucial to have an experienced personal injury attorney by your side to help you navigate the complex process of filing a claim or lawsuit. Your lawyer will be able to determine the true value of your claim and ensure that you recover the maximum compensation possible.
FREE, NO OBLIGATION CASE EVALUATION
Filing a Wrongful Death Lawsuit Due to Contaminated Eye Drops
The contaminated EzriCare and Delsam Pharma eye drops have tragically resulted in several fatalities across the country, leaving grieving families devastated by the loss of their loved ones. If you have lost a family member due to the use of these contaminated eye drops, you may have grounds to pursue a wrongful death lawsuit against the responsible parties.
A wrongful death lawsuit seeks to hold the liable parties accountable for the death of an individual caused by their negligence, recklessness, or intentional actions. In the case of the EzriCare eye drops, Global Pharma, the manufacturer, may be held legally responsible for the harm and fatal consequences resulting from their defective product.
When pursuing a wrongful death lawsuit, surviving family members can seek compensation for various damages, including but not limited to:
- Funeral and burial expenses: Recovering the costs associated with laying a loved one to rest can alleviate the financial burden on the family during this difficult time.
- Medical expenses: Compensation for medical bills incurred prior to the passing of the deceased can be pursued through the lawsuit.
- Loss of financial support: If the deceased was the primary earner for the family, compensation may be sought to cover the loss of income and financial support.
- Loss of companionship: Family members may be entitled to damages for the emotional pain and suffering resulting from the loss of their loved one’s companionship and support.
- Pain and suffering: If the deceased endured pain and suffering before their passing, the family may seek compensation on their behalf.
Filing a wrongful death lawsuit is complex and emotionally challenging. It requires strong legal representation from experienced attorneys who are well-versed in handling such cases. Farahi Law Firm’s compassionate team of lawyers understands the profound impact of losing a loved one due to the negligence of others. We are dedicated to providing personalized support and guidance throughout the legal proceedings.
If you believe you have a potential wrongful death claim related to the contaminated eye drops, it is essential to act promptly. Statutes of limitations may apply, limiting the time within which you can file a lawsuit. By contacting our firm as soon as possible, we can initiate an investigation, gather evidence, and build a strong case to seek justice for your loved one.
Want to file a wrongful death lawsuit due to
contaminated eye drops?
Eye Drop Lawsuit: FAQs
Who Can File an EzriCare Eye Drop Lawsuit?
You may be eligible to file an EzriCare eye drops lawsuit if you or a loved one:
- Used Delsam Pharma or EzriCare Artificial Tears
- Experienced vision loss, eye infections, or sustained other injuries due to the use of these products
It must be noted that you only have a limited amount of time to file a valid claim due to the statute of limitations. Contact us today to find out whether or not you have a case.
How to File an EzriCare Lawsuit
We understand the hardship that you and your family are facing. To make filing an EzriCare eye drop lawsuit simpler for clients, we take charge of every step in the legal process for free – no upfront fees necessary!
With an experienced attorney by your side, you will be able to:
- Verify your eligibility to file a claim
- Gather your medical records and testimony and build a strong case
- File your eye drop lawsuit within the statute of limitations
- Negotiate your claim settlement on your behalf
- Represent you in court if your claim goes to trial.
If you or a loved one have been a victim of the EzriCare and Delsam Pharma eye drop products, you must file a claim to recover the maximum compensation you need and deserve. Therefore, it is in your best interest to have a knowledgeable personal injury attorney by your side to help you through this process.
Our award-winning team of attorneys have recovered millions of dollars in compensation for hundreds of clients. Not only will we handle the complex paperwork, but we will also ensure that you receive the medical care you need.
We work on a contingency fee basis, which means No Fees Unless We Win. Do not hesitate to call us at (866) 755-7917 today for a free case consultation. We are available 24/7!


Get Maximum Compensation.
We Will Help You Fight.
We are an award-winning law firm, we prioritize our clients’ needs. Farahi Law Firm is dedicated to provide protection, assistance, and legal representation to victims of negligent corporations that failed to keep their consumers safe. Your justice is our demand!
In individual or class action, our principles are to defend you and obtain the maximum compensation you deserve. We seek to condemn negligent actions that have harmed innocent people for life and bring peace to their lives.
Copyright © 2023 FARAHI LAW FIRM, APC. All Rights Reserved.
